Benzene Ring Ir Spectrum
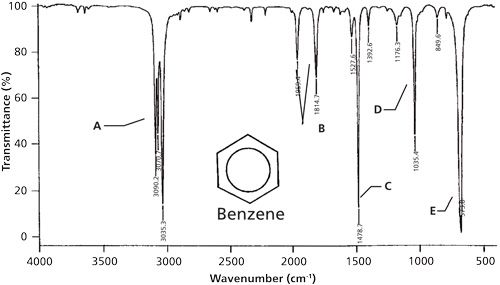
The infrared (IR) spectrum of a benzene ring is a fascinating subject in organic chemistry, offering valuable insights into the vibrational modes of this aromatic compound. Benzene, a cornerstone of aromatic chemistry, exhibits unique IR spectral features that reflect its distinct molecular structure and bonding characteristics. This analysis will delve into the intricacies of the benzene ring’s IR spectrum, exploring the various vibrational modes and their corresponding spectral signatures.
Introduction to Benzene’s Molecular Structure
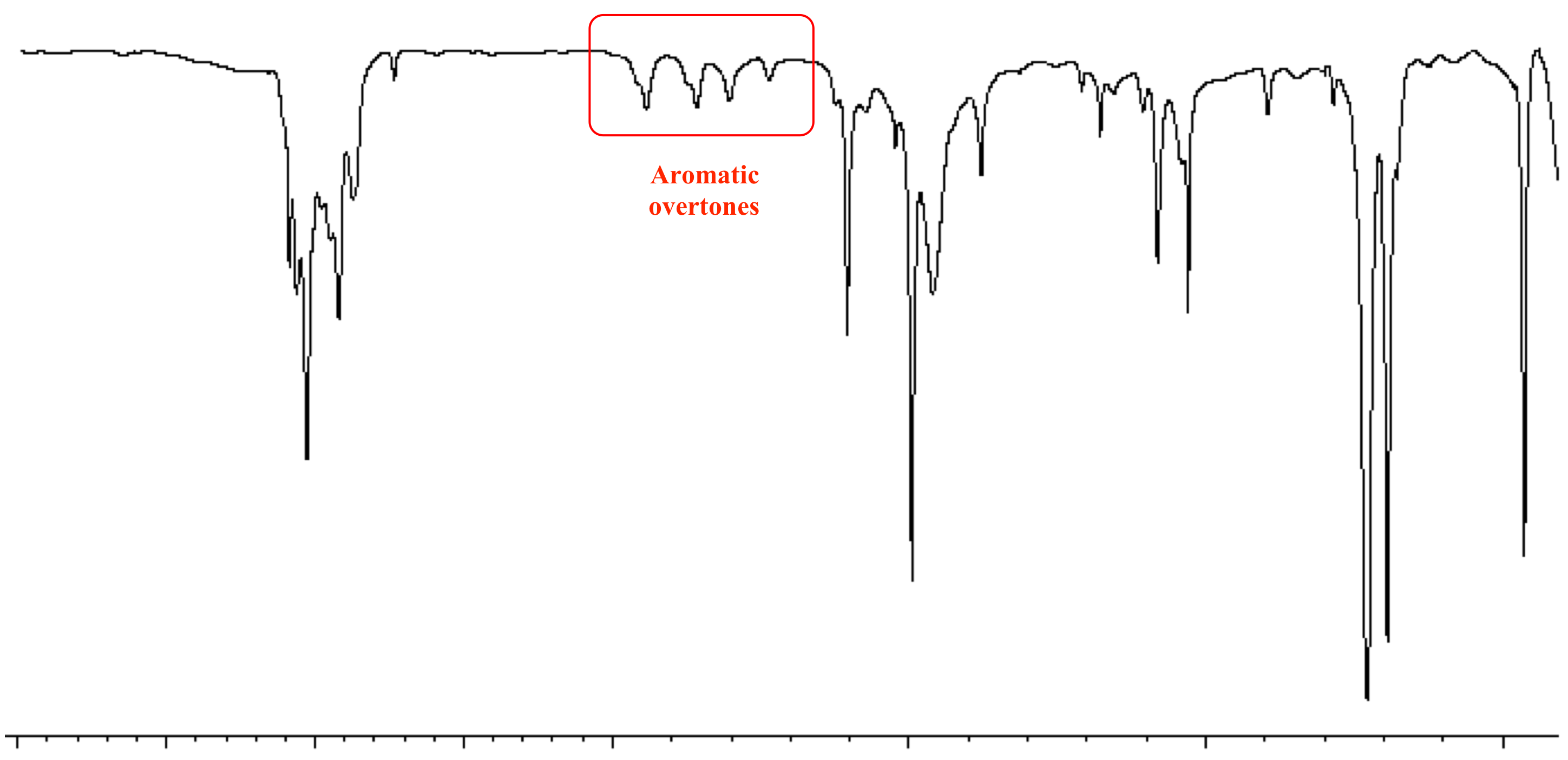
Benzene (C6H6) is a planar, cyclic molecule with six carbon atoms and six hydrogen atoms, arranged in a symmetrical hexagonal shape. The carbon-carbon bonds in benzene are of intermediate length, between those of single and double bonds, due to the delocalized pi electron system. This aromaticity imparts unique chemical and spectral properties to benzene.
Vibrational Modes of Benzene

In the context of IR spectroscopy, molecules absorb infrared radiation that matches the frequency of their natural vibrational modes. Benzene, being a relatively simple molecule, exhibits several distinct vibrational modes, each associated with specific bond movements.
1. C-H Stretching Vibrations
The C-H bonds in benzene undergo stretching vibrations, which are typically observed in the IR spectrum. These vibrations can be further categorized into two types:
- Symmetric C-H Stretch: All six C-H bonds stretch in phase, resulting in a strong absorption band.
- Asymmetric C-H Stretch: Adjacent C-H bonds stretch in opposite directions, leading to a weaker absorption band.
2. C-C Stretching Vibrations
The carbon-carbon bonds in benzene also undergo stretching vibrations, although these are less prominent in the IR spectrum due to the lower polarity change associated with C-C bond stretching.
3. Ring Deformation Modes
Benzene exhibits several ring deformation modes, where the carbon atoms move relative to each other while maintaining the overall planarity of the molecule. These modes include:
- In-plane Bending: Carbon atoms move in the plane of the ring.
- Out-of-plane Bending (OOP): Carbon atoms move perpendicular to the ring plane.
IR Spectrum of Benzene: A Detailed Analysis
The IR spectrum of benzene is characterized by several distinct absorption bands, each corresponding to specific vibrational modes. A typical benzene IR spectrum exhibits the following features:
| Absorption Band (cm-1) | Vibrational Mode | Intensity |
|---|---|---|
| 3050-3100 | C-H Stretching (Symmetric) | Strong |
| 3020-3030 | C-H Stretching (Asymmetric) | Medium |
| 1450-1600 | C-C Stretching and Ring Deformation | Weak to Medium |
| 650-900 | Out-of-Plane Bending | Weak |

Comparative Analysis: Benzene vs. Substituted Benzenes
The IR spectrum of benzene serves as a benchmark for understanding the spectra of its substituted derivatives. When functional groups are introduced onto the benzene ring, the IR spectrum undergoes characteristic changes.
"The introduction of electron-donating or electron-withdrawing groups onto the benzene ring alters the electron density distribution, leading to shifts in vibrational frequencies and changes in band intensities."
1. Electron-Donating Groups (EDGs)
EDGs, such as -OH, -NH2, and -CH3, increase the electron density on the benzene ring, resulting in:
- Blue Shift: C-H stretching vibrations shift to higher frequencies.
- Increased Intensity: C-H stretching bands become more intense.
2. Electron-Withdrawing Groups (EWGs)
EWGs, such as -NO2, -COOH, and -CF3, decrease the electron density on the benzene ring, leading to:
- Red Shift: C-H stretching vibrations shift to lower frequencies.
- Decreased Intensity: C-H stretching bands become less intense.
Practical Applications and Considerations

The IR spectrum of benzene and its derivatives has numerous practical applications in organic chemistry and related fields.
1. Structural Elucidation
IR spectroscopy is a valuable tool for determining the structure of unknown aromatic compounds. By comparing the IR spectrum of an unknown sample to that of known compounds, chemists can identify the presence of aromatic rings and specific functional groups.
2. Purity Assessment
The IR spectrum can also be used to assess the purity of benzene and its derivatives. Impurities or side products often exhibit distinct IR features, allowing for their detection and quantification.
Future Trends and Developments
Advancements in IR spectroscopy technology continue to enhance our understanding of benzene’s vibrational modes and their spectral signatures.
1. High-Resolution IR Spectroscopy
The development of high-resolution IR spectrometers enables the detection of subtle spectral features, improving the accuracy of benzene analysis.
2. Computational Spectroscopy
Computational methods, such as density functional theory (DFT) calculations, allow for the prediction of IR spectra and the assignment of vibrational modes, complementing experimental data.
What is the characteristic IR absorption range for aromatic C-H bonds?
+Aromatic C-H bonds typically exhibit IR absorption bands in the range of 3000-3100 cm-1, with symmetric stretching vibrations appearing as strong peaks.
How does the presence of electron-withdrawing groups affect the IR spectrum of benzene?
+Electron-withdrawing groups cause a red shift in C-H stretching vibrations and decrease their intensity due to the reduced electron density on the benzene ring.
Can IR spectroscopy distinguish between different substitution patterns on the benzene ring?
+Yes, IR spectroscopy can provide valuable information about substitution patterns by analyzing the shifts and intensities of C-H stretching vibrations and other spectral features.
What are the limitations of using IR spectroscopy for benzene analysis?
+IR spectroscopy has limited sensitivity for detecting C-C bond vibrations and can exhibit overlapping bands, which may complicate spectral interpretation. Additionally, it provides no information about the spatial arrangement of substituents on the benzene ring.
How can computational methods enhance the interpretation of benzene IR spectra?
+Computational methods, such as DFT calculations, can predict IR spectra and assign vibrational modes, aiding in the interpretation of experimental data and providing insights into the electronic structure of benzene and its derivatives.
In conclusion, the IR spectrum of the benzene ring is a rich source of information about its vibrational modes and molecular structure. By understanding the characteristic absorption bands and their relationship to specific vibrational modes, chemists can gain valuable insights into the properties and behavior of benzene and its derivatives. As technology continues to advance, our ability to analyze and interpret these spectra will only improve, further expanding our knowledge of aromatic compounds and their applications.