H2 Boiling Point Explained
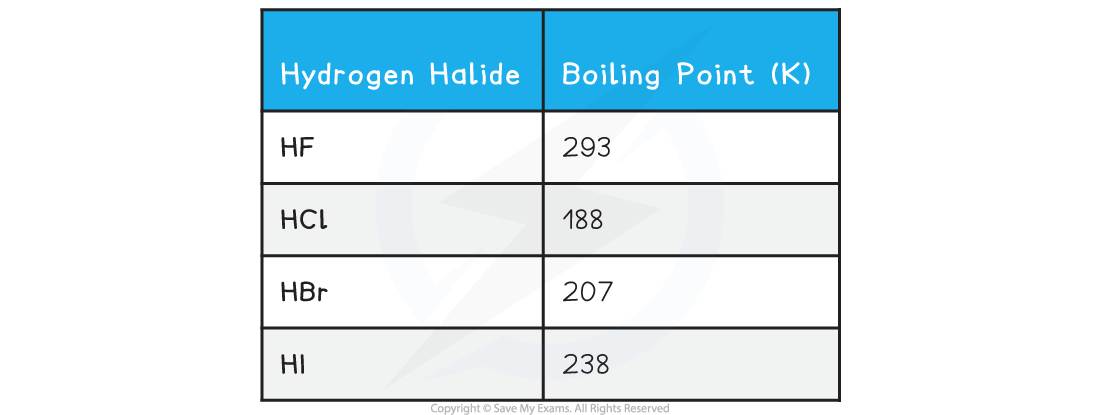
The boiling point of hydrogen gas (H₂) is an intriguing phenomenon that challenges our everyday understanding of how substances behave under heat. At standard atmospheric pressure, hydrogen transitions from a liquid to a gas at an astonishingly low temperature: -252.87°C (-423.17°F). This makes it the substance with the lowest boiling point in the universe, a fact that has profound implications for its storage, transport, and applications across industries.
To put this into perspective, liquid hydrogen exists at temperatures colder than the darkest regions of interstellar space. This extreme cryogenic nature is both a blessing and a challenge, enabling revolutionary technologies while demanding specialized handling.
The Science Behind H₂’s Boiling Point
Hydrogen’s exceptionally low boiling point stems from its unique molecular structure and intermolecular forces. As the simplest diatomic molecule, H₂ consists of two hydrogen atoms bonded by a single covalent bond. Its low mass (approximately 2 atomic mass units) results in minimal kinetic energy requirements for phase transition.
Key Factors Influencing H₂'s Boiling Point:
- Weak Intermolecular Forces: Hydrogen molecules are held together primarily by van der Waals forces, the weakest type of intermolecular attraction. These forces require minimal energy to overcome, allowing H₂ to boil at cryogenic temperatures.
- Low Molar Mass: With a molar mass of just 2 g/mol, hydrogen molecules possess less kinetic energy compared to heavier gases, further reducing the energy needed for phase change.
- Quantum Effects: At such low temperatures, quantum mechanical effects become significant. H₂ molecules exhibit zero-point energy, a residual energy that persists even at absolute zero, influencing their behavior near the boiling point.
Comparative Analysis: H₂ vs. Other Cryogenic Fluids
To understand H₂’s boiling point in context, let’s compare it to other cryogenic fluids:
| Substance | Boiling Point at 1 atm (°C) | Key Applications |
|---|---|---|
| Hydrogen (H₂) | -252.87 | Rocket propulsion, fuel cells, industrial processes |
| Helium (He) | -268.93 | Superconductivity, cryogenics, MRI machines |
| Nitrogen (N₂) | -195.8 | Food preservation, material processing, cryotherapy |
| Oxygen (O₂) | -182.96 | Medical oxygen, industrial combustion, welding |
While hydrogen's boiling point is higher than helium's, its significantly higher energy density and reactivity make it a more versatile cryogenic fluid for energy applications.
Practical Implications of H₂’s Boiling Point
The extreme cryogenic nature of hydrogen presents both opportunities and challenges across various sectors:
Advantages:
- High Energy Density: Hydrogen contains nearly three times the energy of gasoline by weight, making it an ideal candidate for clean energy storage.
- Zero Emissions: When burned or used in fuel cells, hydrogen produces only water vapor, offering a pathway to decarbonize industries.
Challenges:
- Storage Complexity: Maintaining liquid hydrogen requires specialized insulated tanks and continuous cooling to prevent boil-off.
- Safety Concerns: Hydrogen's low boiling point increases the risk of leaks and explosions if not handled properly.
Historical Evolution of H₂ Cryogenics
The study of hydrogen’s boiling point dates back to the late 19th century, when scientists first liquefied gases using Joule-Thomson expansion. James Dewar’s invention of the vacuum flask (Dewar flask) in 1892 revolutionized cryogenic storage, enabling the practical use of liquid hydrogen.
"The liquefaction of hydrogen was a milestone in the history of science, opening doors to advancements in physics, chemistry, and engineering." - Nobel Laureate Heike Kamerlingh Onnes
Future Trends: H₂ in the Energy Transition
As the world shifts toward renewable energy, hydrogen’s unique boiling point properties position it as a cornerstone of the green economy. Emerging technologies like:
- Green Hydrogen Production: Electrolysis powered by renewable energy reduces carbon emissions during production.
- Liquid Hydrogen Carriers: Innovations in storage materials, such as metal-organic frameworks, aim to reduce boil-off losses.
- Cryogenic Fuel Systems: Advances in insulation and refrigeration technology are making hydrogen transport more efficient.
By 2050, the global hydrogen market is projected to reach $200 billion, with liquid hydrogen playing a pivotal role in sectors like aviation, shipping, and heavy industry.
Why is hydrogen's boiling point so low compared to other gases?
+Hydrogen's low boiling point results from its minimal molar mass and weak van der Waals intermolecular forces, requiring less energy to transition from liquid to gas.
Can hydrogen be stored at room temperature?
+No, hydrogen exists as a gas at room temperature and atmospheric pressure. Storing it as a liquid requires temperatures below -252.87°C.
What is boil-off in liquid hydrogen storage?
+Boil-off refers to the continuous evaporation of liquid hydrogen due to heat leakage, even in well-insulated storage systems. This loss must be managed through venting or re-liquefaction.
How does hydrogen's boiling point impact its use in fuel cells?
+Fuel cells typically use gaseous hydrogen, which is easier to handle than liquid hydrogen. However, liquid hydrogen's high energy density makes it ideal for applications requiring compact storage, like long-haul transportation.
Hydrogen’s boiling point is more than a scientific curiosity—it’s a critical factor shaping its role in the future of energy. As technology advances, overcoming the challenges associated with its cryogenic nature will unlock unprecedented opportunities for a sustainable world.
